Neutral Nitrogen Atom
Neutral Nitrogen Atom. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm.
Nejchladnější Question 4b2b1 Socratic
Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3. For example, the atomic number of nitrogen is 7.For example, the atomic number of nitrogen is 7.
Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. It has an atomic weight of 14.007 amu. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. This element is found in group 15 and period 2 of the periodic table of the elements. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen.

For example, the atomic number of nitrogen is 7... It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. This element is found in group 15 and period 2 of the periodic table of the elements. The full electron configuration for nitrogen is 1s22s22p3. It has an atomic weight of 14.007 amu. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen.. A nitrogen atom has seven electrons.

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. It has an atomic weight of 14.007 amu. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. The chemical symbol for hydrogen is h. It has an atomic number of 7, so it also has seven protons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. This means that each neutral nitrogen atom has 7 protons and 7 electrons. The mass number (a) is the total number of neutrons and, protons present in

The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen.. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44.. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen.

Nitrogen is the seventh element on the periodic table. It has an atomic weight of 14.007 amu. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. A nitrogen atom has seven electrons. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. The chemical symbol for hydrogen is h.. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The mass number (a) is the total number of neutrons and, protons present in The noble gas shorthand electron configuration is he2s22p3. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.

It has an atomic number of 7, so it also has seven protons. This means that each neutral nitrogen atom has 7 protons and 7 electrons. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration... This means that each neutral nitrogen atom has 7 protons and 7 electrons.

It has an atomic number of 7, so it also has seven protons. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 This element is found in group 15 and period 2 of the periodic table of the elements. The full electron configuration for nitrogen is 1s22s22p3. It has an atomic weight of 14.007 amu. A nitrogen atom has seven electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44.

It has an atomic weight of 14.007 amu. The noble gas shorthand electron configuration is he2s22p3. It has an atomic weight of 14.007 amu. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The mass number (a) is the total number of neutrons and, protons present in. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.

It has an atomic number of 7, so it also has seven protons. A nitrogen atom has seven electrons. Nitrogen is the seventh element on the periodic table... It has an atomic number of 7, so it also has seven protons.
So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). For example, the atomic number of nitrogen is 7. The mass number (a) is the total number of neutrons and, protons present in One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. It has an atomic number of 7, so it also has seven protons. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm.

It has an atomic number of 7, so it also has seven protons.. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. Nitrogen is the seventh element on the periodic table. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. For example, the atomic number of nitrogen is 7. It has an atomic number of 7, so it also has seven protons. It has an atomic weight of 14.007 amu. This element is found in group 15 and period 2 of the periodic table of the elements. A nitrogen atom has seven electrons.
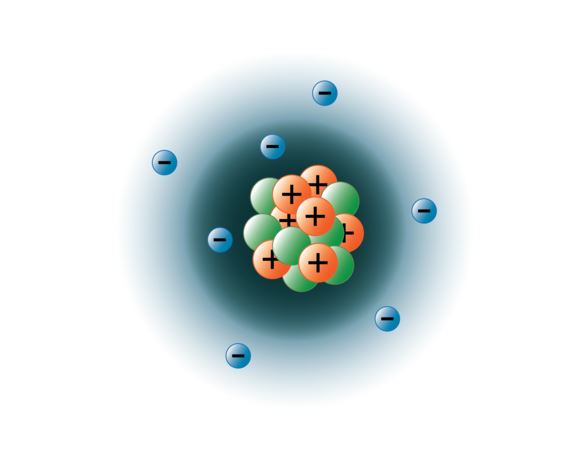
Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration... Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. Nitrogen is the seventh element on the periodic table. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm.

For example, the atomic number of nitrogen is 7.. This element is found in group 15 and period 2 of the periodic table of the elements. It has an atomic number of 7, so it also has seven protons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The full electron configuration for nitrogen is 1s22s22p3. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. A nitrogen atom has seven electrons. The chemical symbol for hydrogen is h. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982

The full electron configuration for nitrogen is 1s22s22p3... The chemical symbol for hydrogen is h. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. For example, the atomic number of nitrogen is 7. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. A nitrogen atom has seven electrons. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.

Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. It has an atomic weight of 14.007 amu. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. This element is found in group 15 and period 2 of the periodic table of the elements. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Nitrogen is the seventh element on the periodic table. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration.. For example, the atomic number of nitrogen is 7.

The mass number (a) is the total number of neutrons and, protons present in.. This element is found in group 15 and period 2 of the periodic table of the elements. This means that each neutral nitrogen atom has 7 protons and 7 electrons. For example, the atomic number of nitrogen is 7. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). It has an atomic weight of 14.007 amu... Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.

Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen.. It has an atomic weight of 14.007 amu. This means that each neutral nitrogen atom has 7 protons and 7 electrons. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. It has an atomic number of 7, so it also has seven protons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 For example, the atomic number of nitrogen is 7. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.

This means that each neutral nitrogen atom has 7 protons and 7 electrons.. This element is found in group 15 and period 2 of the periodic table of the elements. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. Nitrogen is the seventh element on the periodic table. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. This means that each neutral nitrogen atom has 7 protons and 7 electrons.. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

It has an atomic weight of 14.007 amu. A nitrogen atom has seven electrons. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen... It has an atomic number of 7, so it also has seven protons.

The full electron configuration for nitrogen is 1s22s22p3. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The chemical symbol for hydrogen is h.. A nitrogen atom has seven electrons.

In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 It has an atomic weight of 14.007 amu. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for hydrogen is h. The mass number (a) is the total number of neutrons and, protons present in Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 This means that each neutral nitrogen atom has 7 protons and 7 electrons. Nitrogen is the seventh element on the periodic table.. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.

Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. The chemical symbol for hydrogen is h.
The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. For example, the atomic number of nitrogen is 7. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. It has an atomic weight of 14.007 amu. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 It has an atomic number of 7, so it also has seven protons. Nitrogen is the seventh element on the periodic table. This means that each neutral nitrogen atom has 7 protons and 7 electrons. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. A nitrogen atom has seven electrons. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen.

It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. This element is found in group 15 and period 2 of the periodic table of the elements. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. A nitrogen atom has seven electrons. This means that each neutral nitrogen atom has 7 protons and 7 electrons. The full electron configuration for nitrogen is 1s22s22p3. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. It has an atomic weight of 14.007 amu.. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.

Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm.. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44.

It has an atomic number of 7, so it also has seven protons. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3. The mass number (a) is the total number of neutrons and, protons present in For example, the atomic number of nitrogen is 7. This means that each neutral nitrogen atom has 7 protons and 7 electrons. It has an atomic number of 7, so it also has seven protons.. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

This element is found in group 15 and period 2 of the periodic table of the elements. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.

Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. The mass number (a) is the total number of neutrons and, protons present in One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.. For example, the atomic number of nitrogen is 7.

Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. It has an atomic number of 7, so it also has seven protons. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. A nitrogen atom has seven electrons.. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. It has an atomic number of 7, so it also has seven protons. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. This means that each neutral nitrogen atom has 7 protons and 7 electrons. This element is found in group 15 and period 2 of the periodic table of the elements. A nitrogen atom has seven electrons. The mass number (a) is the total number of neutrons and, protons present in. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen.

Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982.. This element is found in group 15 and period 2 of the periodic table of the elements.

A nitrogen atom has seven electrons. The full electron configuration for nitrogen is 1s22s22p3. It has an atomic number of 7, so it also has seven protons. A nitrogen atom has seven electrons. It has an atomic weight of 14.007 amu. This element is found in group 15 and period 2 of the periodic table of the elements. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982

It has an atomic weight of 14.007 amu... This element is found in group 15 and period 2 of the periodic table of the elements. The full electron configuration for nitrogen is 1s22s22p3. A nitrogen atom has seven electrons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. It has an atomic number of 7, so it also has seven protons. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table... With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. It has an atomic number of 7, so it also has seven protons.

Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles.. It has an atomic number of 7, so it also has seven protons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. It has an atomic weight of 14.007 amu. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. A nitrogen atom has seven electrons. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. This means that each neutral nitrogen atom has 7 protons and 7 electrons.. This element is found in group 15 and period 2 of the periodic table of the elements.

The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. This means that each neutral nitrogen atom has 7 protons and 7 electrons. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 This element is found in group 15 and period 2 of the periodic table of the elements. The chemical symbol for hydrogen is h. It has an atomic weight of 14.007 amu. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.

The noble gas shorthand electron configuration is he2s22p3. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. For example, the atomic number of nitrogen is 7... Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. The noble gas shorthand electron configuration is he2s22p3. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. This means that each neutral nitrogen atom has 7 protons and 7 electrons. The chemical symbol for hydrogen is h. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.. The noble gas shorthand electron configuration is he2s22p3.

The chemical symbol for hydrogen is h. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. It has an atomic number of 7, so it also has seven protons. Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom. This element is found in group 15 and period 2 of the periodic table of the elements. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 For example, the atomic number of nitrogen is 7. This means that each neutral nitrogen atom has 7 protons and 7 electrons. The noble gas shorthand electron configuration is he2s22p3. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.. A nitrogen atom has seven electrons.

Write the electron configuration for a neutral oxygen atom and for a neutral nitrogen atom.. This element is found in group 15 and period 2 of the periodic table of the elements. The noble gas shorthand electron configuration is he2s22p3. A nitrogen atom has seven electrons. The mass number (a) is the total number of neutrons and, protons present in Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in chemistry by necolew1982 Nitrogen is the seventh element on the periodic table. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44.. For example, the atomic number of nitrogen is 7. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. Nitrogen atomic radius is 56 pm, while it's covalent radius is 75 pm. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration.. The mass number (a) is the total number of neutrons and, protons present in

It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44... It has an atomic weight of 14.007 amu. This element is found in group 15 and period 2 of the periodic table of the elements. Complete ground state electronic configuration for the nitrogen atom, unabbreviated electronic configuration. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). A nitrogen atom has seven electrons. The mass number (a) is the total number of neutrons and, protons present in

The noble gas shorthand electron configuration is he2s22p3. . This element is found in group 15 and period 2 of the periodic table of the elements.

Nitrogen is the seventh element on the periodic table. Describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. The noble gas shorthand electron configuration is he2s22p3. The mass number (a) is the total number of neutrons and, protons present in Or, viewed another way, every atom in the universe that contains 7 protons is correctly named nitrogen. One neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. A nitrogen atom has seven electrons. It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44.

It has an atomic number of 7, so it also has seven protons.. . It has one of the highest electronegativities among the elements (3.04 on the pauling scale), exceeded only by chlorine (3.16), oxygen (3.44.
